HIV Assembly and Maturation
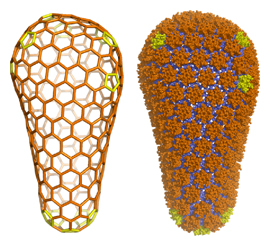
HIV and other orthoretroviruses are membrane-enveloped viruses that adopt two distinct morphological states in the course of their replication. These viruses initially assemble in an immature, non-infectious form, wherein the viral genome is encased within a spherical capsid shell composed of the viral Gag protein. To become infectious, the immature virus undergoes a morphological change into the mature form, wherein the genome is now packaged within a cone-shaped mature capsid composed of the CA protein. This process, termed “maturation,” prepares the genome for delivery into host cells and a new round of replication. Maturation is triggered by proteolytic processing of Gag into CA and other mature proteins, and is a proven target for inhibition of HIV/AIDS in the clinic.
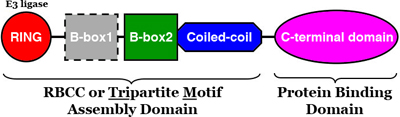
We have previously defined the atomic-resolution structures of the immature and mature capsids of HIV, which described how molecular switches control their structural transformations. We are now studying how small molecules called "maturation inhibitors" disrupt these transformations. Other projects in this area have the broad goal of understanding how host proteins - such as cyclophilins, nucleoporins and TRIMs - recognize the immature and mature capsids in order to positively or negatively regulate specific steps in the HIV-1 life cycle.